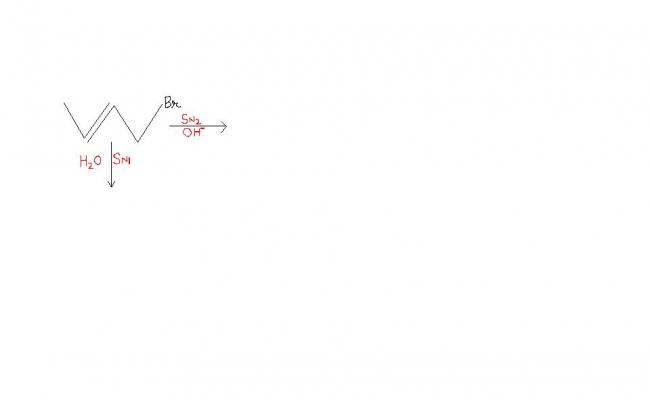
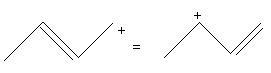it was told by my teacher and i forgot what he said...........
13 Answers
I don't think the carbocation formed is stable. Even a hydride shift won't do it too much good. Do you know the product?
SN1 because of the formation of allylic carbocation which is stablized by resonance
Oh no. I thought the line leading out from the 4th carbon from the left was another CH2Br group. Thanx bhaiyyah. [1]
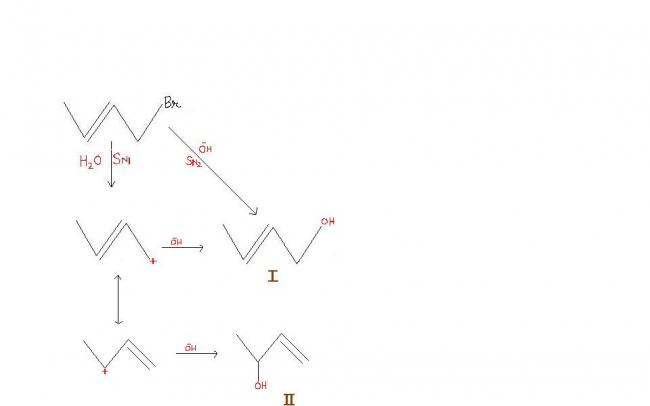
sorry guys ,, actually i have asked wrong question
i was told that in the mechanism product 2 is faster formed....
can anybody tell me the reason ?????
i am extremely sorry for my mistake
reacn 2's carbocation is 2° allylic carbocation thats why is more stable than reaction 1's 1° allylic carbocation
I think it will depend on solvents and relative rates as I is being formed via 2 paths and II via a single path and we have to decide which will dominate..and whether the stability of carbocation increases rate of reaction so as to overtake the sum of two individual paths...That depends on how much is it stable...
But this is chemistry.. If U gives I is formed at faster rate or II is faster both can be wrong it will depend on examiner to decide which is correct and whatever he says is Brahmalakir