activation energy for substitution rxns are low when compared to elimination reactions
why substitution reactions favoured at low temp?????????
-
UP 0 DOWN 0 0 6

6 Answers
msp
·2009-02-18 08:12:16
Abhishek Priyam
·2009-02-18 08:12:54
because energy of activation is less for substitution reaction...
vector
·2009-02-18 08:14:50
but with increase in temp why d rates decrease?
y elimination favoured over substitution?
Abhishek Priyam
·2009-02-18 08:25:35
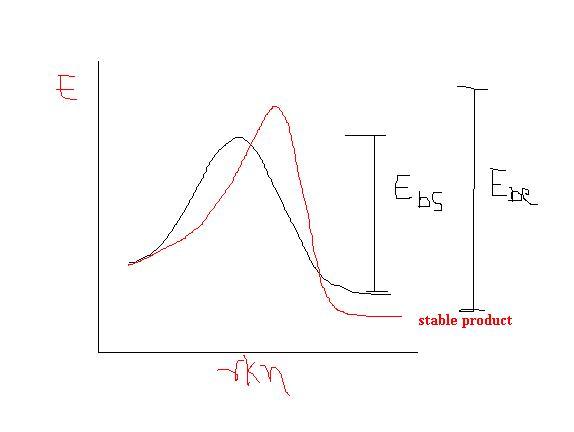
If temp is increased.. sn product is formed but as E back of SN is less so less stable SN product is converted backwards and then Elimination product is formed but as E back of elim product is greater so no backward from elimination product hence in long run Elim product dominates...
At start of reaction (first second of rkn..)SN product is greater but finally E product increaees...)