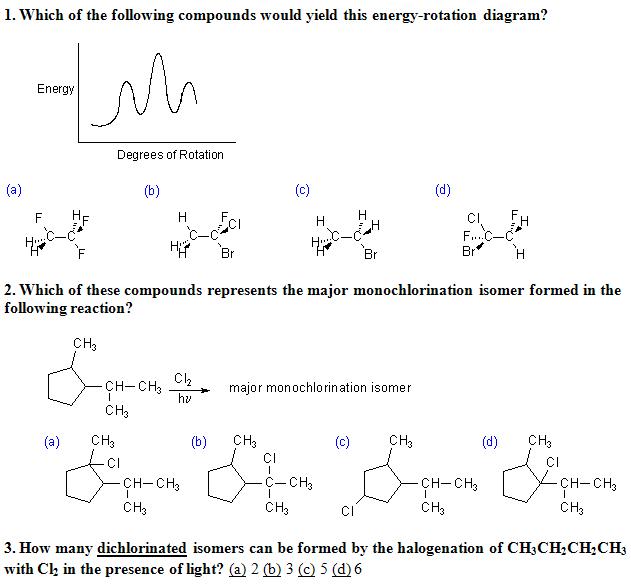
2)
3° radical is more stable naa.....so answer should be b
20 Answers
yes sir i agree that there were six levels,i thot of relative heights of minima so i guessed it shud be d,
Best Bet is (a) because for that 4 energy levels are possible.
For (b) and (c) 2 energy levels are possible
For(d) 6 energy levels are possible.
is it (a) because a lot of H-bonding is ther which makes the given str more stable while rotation would decrease stability as H-bonding would be reduced??
not done perfectly but first look
But in (b) there will be 7 hyperconjugation forms which is greatest from others.
why sir (b) pls see #5 regarding that.
isnt 2° abstraction = 3.8 and as there 2 H... it should be 7.6...
while for 3° it is 1 but only 1 H is there
For second, think which of the intermediate radical will be most stable?
3-(d)
1,1 dichloro butane
2,2 dichloro butane
1,2 dichloro butane
1,3 " " "
1,4
2,3
manish sir, shouldnt 2 be all the three types of mono chloro formed by the replacement of H on secondary carbons on the cyclopentane ring?