consider the compound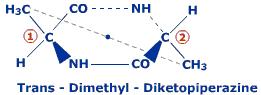
given at
http://www.tutorvista.com/content/chemistry/chemistry-iv/stereochemistry/symmetric-elements.php
where the dot is centre of symmetry ....so is dis rule applicable for all compounds ??
We know dat a Compound isnt optically active if it has
1] plane of symmetry
2]axis of symmetry
3]centre of symmetry
but how do we find whether a compound has any kind of symmetry or not ?? plane of symmetry is not difficult to trace ....but i m finding it difficult wit the other types of symmetry ...so pls can n e 1 give me the rules of how to find any kind of symmetry in a compound with some good examples ???
tnx in advance [1]
consider the compound
given at
http://www.tutorvista.com/content/chemistry/chemistry-iv/stereochemistry/symmetric-elements.php
where the dot is centre of symmetry ....so is dis rule applicable for all compounds ??
A particular example from my notes -: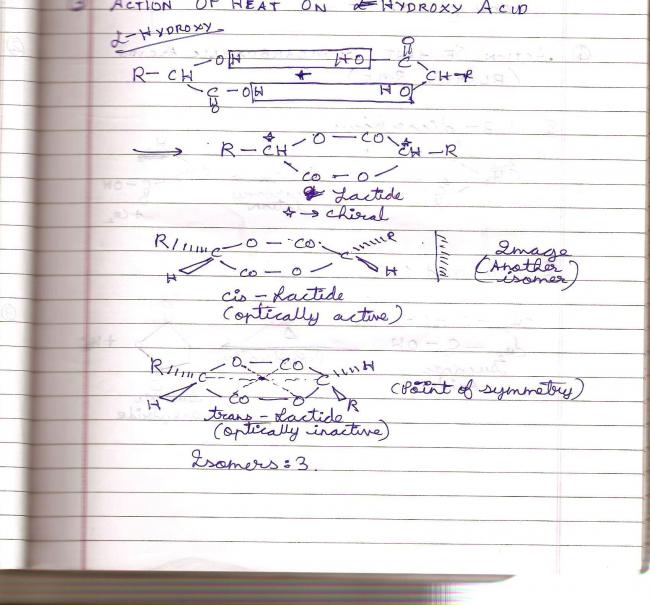
There are 3 isomers formed on heating this alpha hydroxy acid, of which the cis isomer is optically active and has an enantiomer. The trans lactide is optically inactive due to a point of symmetry. Some people confuse having that C=O group means no symmetry, but that's wrong.
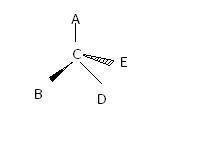
in dis comp if we rotate it by 180 degrees....how is it appearing 2 times?
and if it has even no. of fold axis of symmetry then it is optically inactive na ?wats d main concept behind axis of symm?
i kno some of u r thinking that i always ask simple and rubbish doubts [3]
Going through old posts on tiit always helps......[1]
see these links ...
http://targetiit.com/iit-jee-forum/posts/some-concept-related-to-stereoisomers-4032.html
http://targetiit.com/iit-jee-forum/posts/he-bhagwan-ye-org-3446.html
http://targetiit.com/iit-jee-forum/posts/optical-isomerism-2070.html