actuaaly in optins it is given CH3COCl +LiALH4 .
will it work ?
no i am not talking abt the holllywood movie...
here are two transformtaions...
carry them out using proper reagents
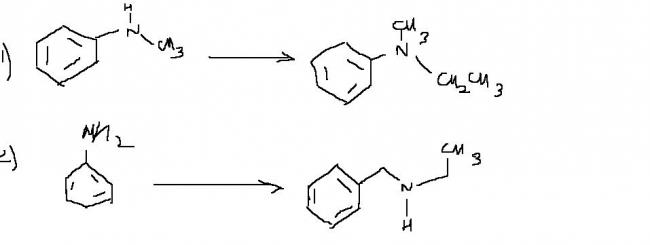
-
UP 0 DOWN 0 0 14

14 Answers
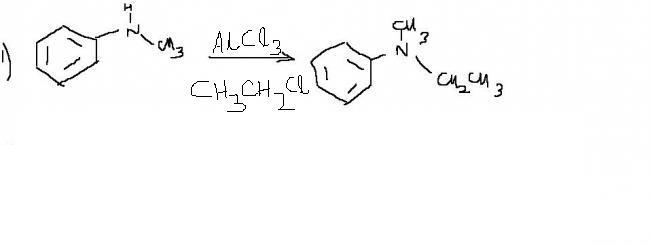
simple way fr 1st one. add AlCl3 + CH3CH2Cl.
thus , AlCl4- + CH3CH2+ are formed. As "N" is more elctronegative than "C" ,
thus , CH3CH2+ attacks amino group. ( similar mechanism as fridel crafts )
debo , i think "LEUCKART" reaction involves a carbonyl group. please make it clear !
ya , it'll do. but it involves bond cleavage , then addition of -CH3CO group and then reduction to alkanes by LiAlH4 . it'll require some amt. of energy.
Q.2) This is a special type of reaction.
u may be confused of this being a nucleophilic substitution reaction in an aromatic ring.
but, this reaction is concerted i.e. no specific reaction intermediate just like SN2. thats why this doesn't form carbocation as the intermediate. rather as soon as the N2+ is eliminated, the nucleophile gets added to it.
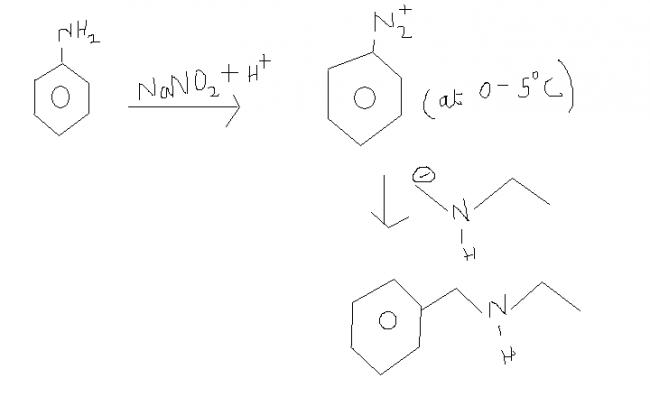
some points abt this reaction :
i) this reaction takes place at low temperature.
ii) this reaction doesn't involve carbocation as the reaction intermediate.
iii) the product formed after 1st step is benzene diazonium ion.
iv) this benzene diazonium ion is the only aromatic ion showing nucleophilic substitution.
I did it in a bit diff way..
1)NaNO2/HCl/0°C
2)CuCN
3)LiAlH4
4)CH3COCl
5)LiAlH4
About the Eschweiler-Clarke reaction,

Source : http://www.organic-chemistry.org
I didn't know REDUCTIVE AMMINATION is this reaction itself!