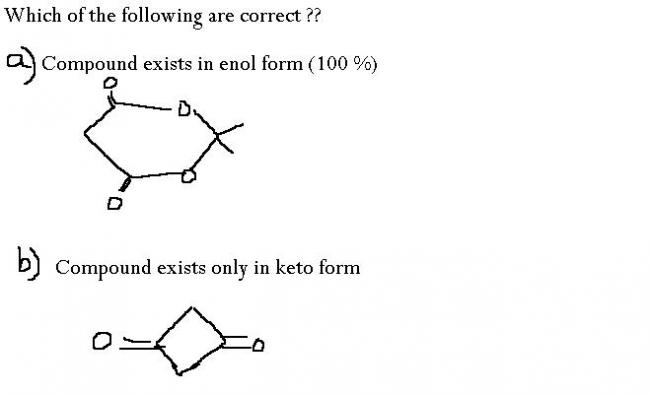
Just blurting out my mind...b) is correct and a) is not.
4 Answers
ronak agarwal
·2010-03-02 09:05:39
i think both r true....
a)enol form of the compound aromatic so the stability increases....
b)enol form makes it anti aromatic....so it will prefer to stay in keto form only
govind
·2010-03-03 03:33:13
For the second ..if u draw the enol form then u will find that the compound has two double bonds which have conjugation ..but since the compound formed is antiaromatic so it be very unstable...that's why no enol form in this case...moreover Bayer strain will also be there in that ring...