Oh got it, rkn in Q1 is Perkin Rkn type.
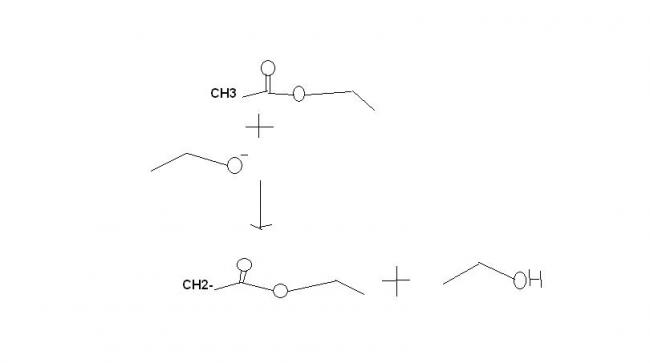
1) CH3COOC2H5 + C2H5ONa → (A) → iodoform reaction → (B) + (C)
2) correct order of increasing rate of hydrolysis of the foll compounds : (gv reasons)
i) CH3COOEt ii) C2H5COOEt iii) iso-propyl-COOEt
3) when propanoic acid is treated with aqueous sodium bicarbonate, CO2 is liberated..
the CO2 comes from ---
i) COOH group
ii) methylene group
iii) bicarbonate
iv) methyl group
-
UP 0 DOWN 0 0 20

20 Answers
I said perkin rkn type, not perkin rkn, i forgot its name, what is there in name when reactants are acid anhydride call it perkin and when ester call it claisen condensation..........
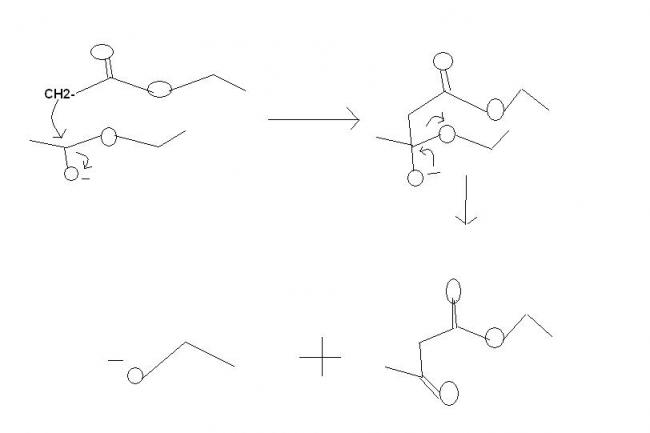
It's not perkin reaction . It's claisen condensation for synthesis of β-ketoesters. the mechanisms fine.
I think that you're answer is rihgt . Which book? If it's Arihant don't trust the solns. w.r.t the rate of hydrolysis.
Hydrolysis is generally governed by steric factors , than polar effects.
For acid hydrolysis , that is the answer . If it's base catalysed , Claisen condensation product if definitely major.
In that also , your order for hydrolysis is fine.
In Q.1
A is CH3COCH2COOC2H5
B is Na+-OOCCH2COOC2H5
C is CHI3
In Q.3
CO2 comes from NaHCO3
CH3CH2COOH+NaHCO3→CH3COO-+Na+CO2+H2O
dats y i posted this question...
coz if some confident banda gives n ans,,, i can assure myself that one given in book is wrong
ok
but i am sry to say... u ARE away from correct ans...
i too thot like you,,,,, but the ans is 3>2>1.
sky .. unfortunately, i am not good enuf in chemistry... unfortunately, I cant give "confident" answers to this one...
I may sometimes (actually more than that) be far far from the correct answer :)
But i will get the other guys to the task :)
Q.2 (considering base catalysed hydrolysis)
i)>ii)>iii)
consider electron density on carbonyl carbon and steric hindranceon adjacent carbon.
HEY Y NONE OF U GUYS ARE ANSWERING THIS ONE???
THESE ARE NOT AT ALL TOUGH!!
I JUS' WANNA SEE IF I AM RIGHT OR WRONG....