71
71--
Also, Name for IUPAC compounds.
6. 2,3-dimethylbicyclo[2.2.0]hexane
4. 2,5-dicyclobutyl-1-methylbicyclo[2.1.0]pentane
2. Unclear.
Please note that I didn't write these names my self. These type of questions are just irrititating and useless enough to be avoided completely. Don't stick into these type of sums. Do genuine sums. These are mindlessly drawn/copied to tease students.
 262
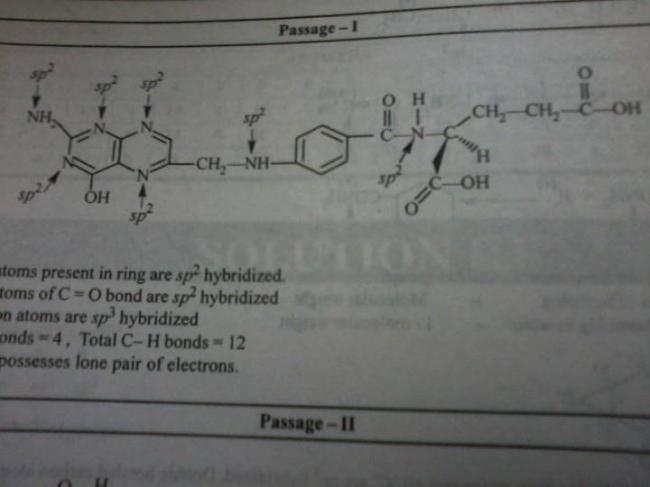
262the hyridizations are correct
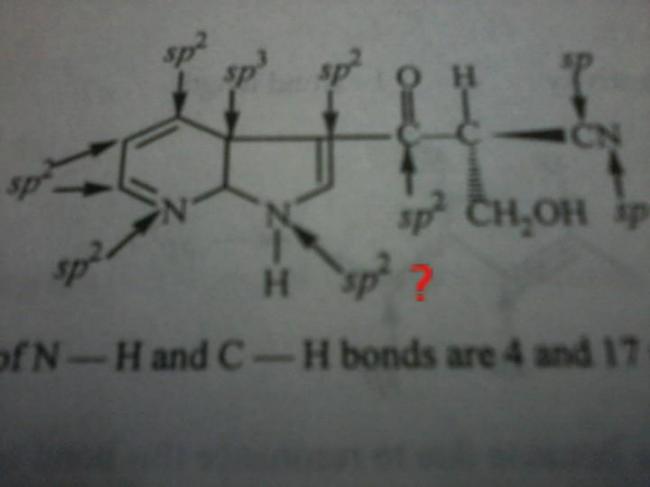
notice that a lone pair will be in resonance , in all above given cases.
hence sp2
 71
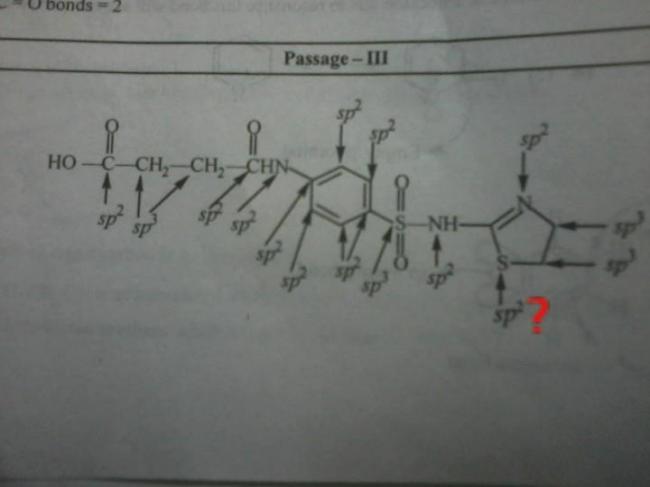
71Fist case where there is S atom.. I'm not getting cconvinced.
 262
262S has two single bonds and one lone pair available (other lone pair is unavailable due to resonance)
hence sp2
 1
1yeah I think Aditya is right....
I think I got answers for all doubts. thanks to Both of you!