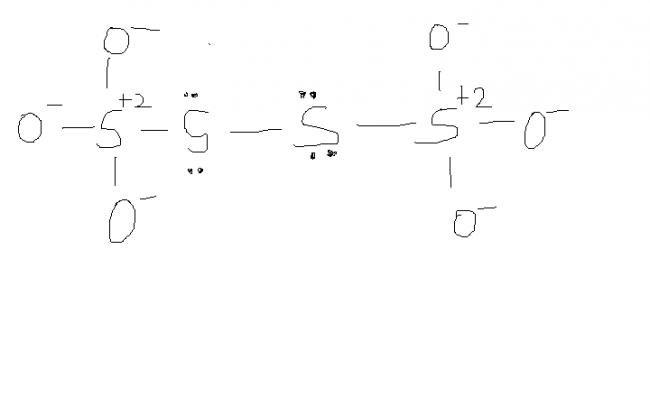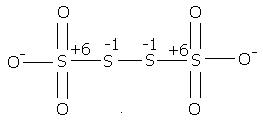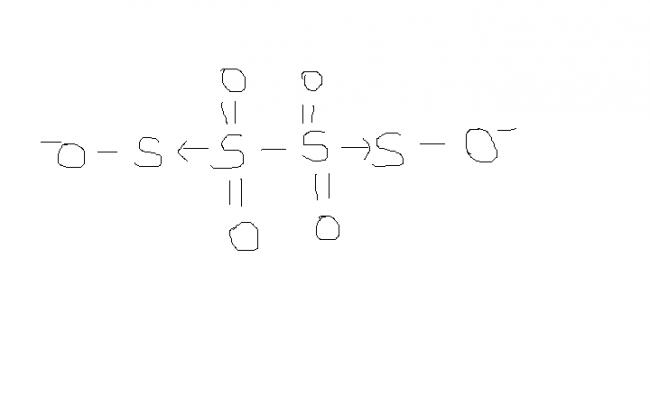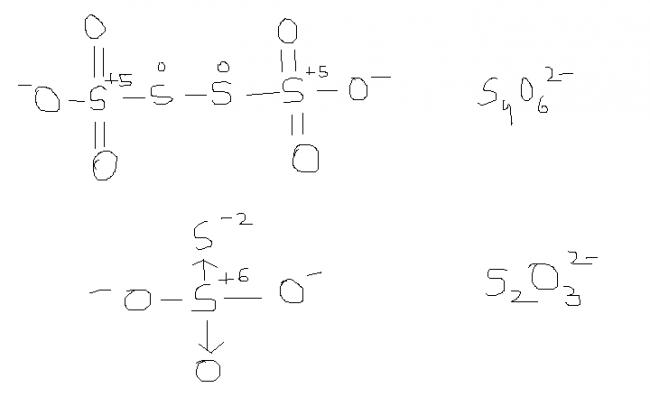1) A solution of 0.2 gm of a compound containing Cu2+ and C2O42- ions on titration with 0.02 M KMnO4 in the presence of H2SO4 consumes 22.6 ml of the oxidant. The resulting solution is neutralized with Na2CO3, acidified with dil. acetic acid and treated with excess KI. The liberated iodine requires 11.3 ml 0.05 M Na2S2O3 for complete reduction, find out the mole ratio of Cu2+ to C2O42- in the compound
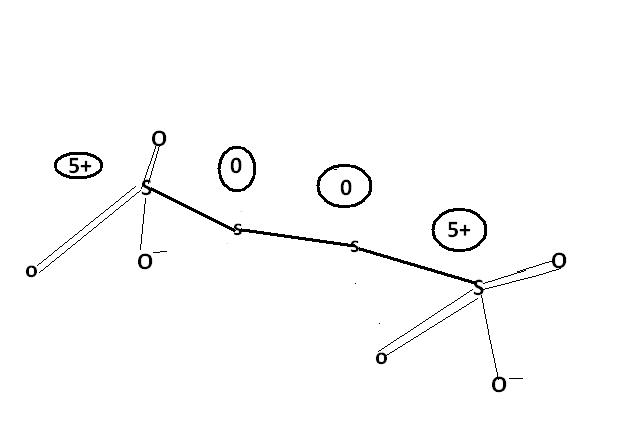
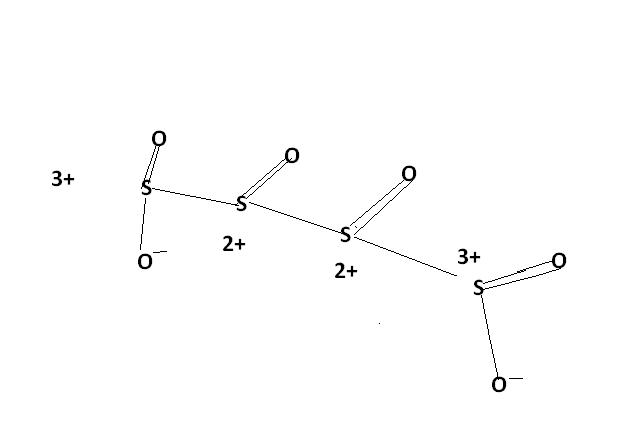
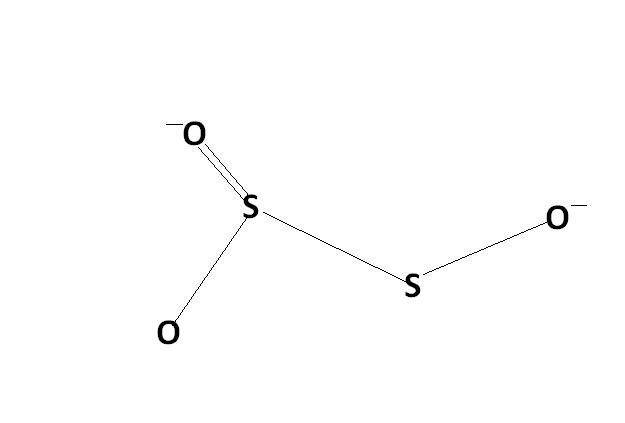
2) find the oxidation number of each sulphur atom in Na2S2O3 and Na2S4O6
3) Balance the following using oxidation number method:
FeS2+O2→Fe2O3+SO2
4) The electronic configuration of an element is 1s22s2......3p63d5. This represents its
(a) excited state (b) ground state (c) cationic form (d) anionic form
5)The velocity of electron in a certain Bohr’s orbit of hydrogen atom bears the ratio of 1 : 275 to the velocity of light
a) What is the quantum number (n) of orbit ?
b) Calculate the wave number of radiations emitted when electron jumps from (n+1) state to ground state
6) A 40 ml solution of weak base BOH is titrated with 0.1 N HCl solution. The pH of the solution is found to be 10.04 and 9.14 after adding 5 ml and 20 ml of the acid respectively. Find the dissociation constant of base
7) A sample of AgCl was treated with 5 ml of 1.5 M Na2CO3 solution to give Ag2CO3. The remaining solution contained 0.0026 g of Cl- per litre. Calculate the solubility product of AgCl (Ksp(Ag2CO3)=8.2×10-12)