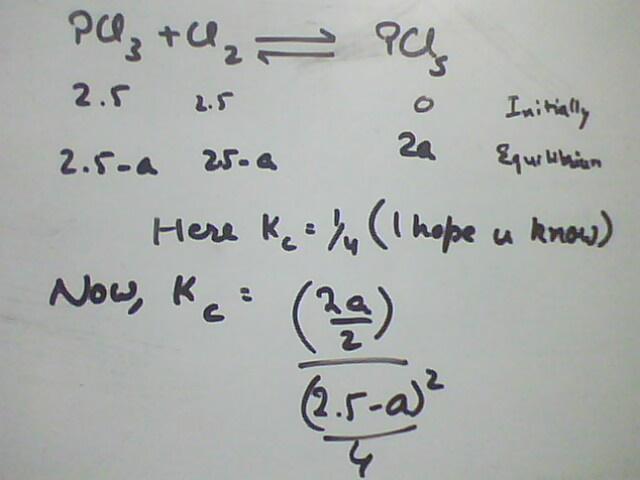1
1arey ni i did that also
teko jo answer mila woh options mein ni hai
optionz r
.25
.125
.75.............. (mera iske karib hai)
1
 11
11SORRY MADE A BLUNDER EARLIER
VOLUME KO 4 LE LIYA THE
I AM GETTING 0.25
 106
106PCl5 = PCl3 + Cl2
0 2.5 2.5
a 2.5-a 2.5-a at equilibrium
So, 4 = [(2.5-a)/2]2/(a/2)
==> 8a = 25/4 + a2 - 5a
==> a2 - 13a + 25/4 = 0
==> 4a2 - 52a + 25 = 0
==> a = (52±48)/8 and + sign can be ignored bcz .. a<2.5
==> a = 4/8 = 0.5 ??
so equilibrium conc of PCl5 at equilibrium = a/2 = 0.5/2 = 0.25
 106
106@mani maine volume ko divide kiya hai
 1
1haan ji thanks answer ni pata bansal notes se hai
answer key ni hai
 1
1@mrunal, v can do it by the reverse process too.......
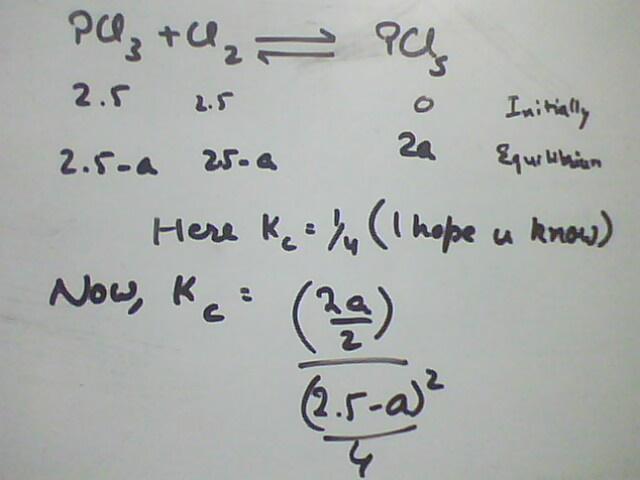
 1
1haan ji i noe dat i did dat
neways thanks :P
btw aap akun ho ?
 1
1i m scientist...[3]
@nishu bhaiya, pink milega??????[7][4][3]
 1357
1357@ scientist.
you have made a small but huge mistake
At equilibrium
As coefficient of all the terms are 1
if 'a' mole of PCl3 is gone then only 'a' mole of PCl5 is formed
How 2a is formed in your case