@ Manish Sir
A-Q
B-S
C-P
D-R
How can D=R??
as shown by you it must be constant
Match the column
Column 1
[A] 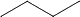 (g) →
(g) →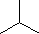 (g)
(g)
[B] I2(s) → I2(aq)
[C] N2O4(g) → 2NO2(g)
[D] AgI(s) → Ag+(aq) + I-(aq)
Column 2
[P]
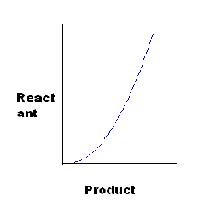
[Q]
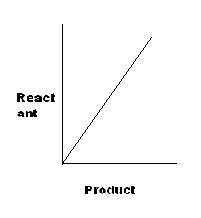
[R]
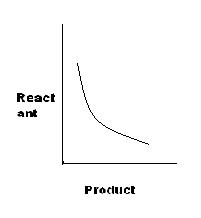
[S]
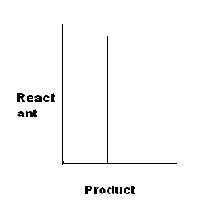
Note: "→" represents an reversible reaction.
Let the concentration of product be x and that of reactant be y.
For A, K=x/y, or y=K'x
for C, K=x2/y, or x2=Ky
For D, K=x.x=x2, or x=K'
For B, I have to search.
@ Manish Sir
A-Q
B-S
C-P
D-R
How can D=R??
as shown by you it must be constant
yeah D should be S only
I have confusion for that physical equilibrium process(melting one)
Ok..I thought its an easy one..As i m not good in chemistry..Anyways thank u guys..Atleast some of you tried))