i agree with ashish's answer...hes bang on target
Q : 2 H2 + O2 → 2 H2O
This reaction is exothermic in the forward direction:
2 H2 + O2 → 2 H2O + 285 kJ
but endothermic in the reverse direction:
2 H2O + 285 kJ → 2 H2 + O2
Consider a general reversible reaction such as:
A + B → C + D
Given the following potential energy diagram for this reaction, determine ΔH and Ea for
both the forward and reverse directions. Is the forward reaction endothermic or exothermic?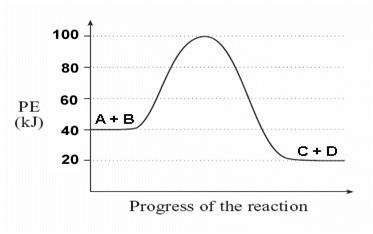
-
UP 0 DOWN 0 0 2

2 Answers
Asish Mahapatra
·2009-12-21 21:32:25
Forward reaction:
ΔH = -20 kJ
Ea = 60 kJ
exothermic
Reverse reaction:
ΔH = +20 kJ
Ea = 80 kJ
endothermic