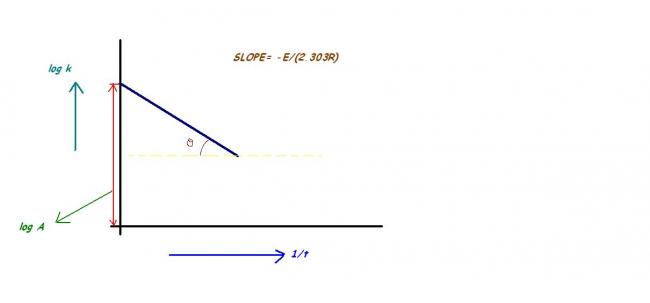
plot of log k versus time 1/t for first order reaction
-
UP 0 DOWN 0 0 3

3 Answers
Lokesh Verma
·2009-07-24 00:05:44
sumeet this is another standard questions...
try to use kt=A.e-Ea/RT
Take log of the expression and see what happens..