@shahrukh
for boiling point the reason is easy and understandable but can you please explain in a more lenthy way for freezing point depression
Q1- Can someone please tell me the exact and well described reason for depression of freezing point on addition of non volatile solute to a solvent?
Q2- How does the vapour pressure of a pure solution change when we increase or decrease the external pressure or internal pressure of the solution?
Q3- Two beakers one containing 0.01 mole of sugar in 90g of water and other containing 0.02 mole of sugar in 90g of water are kept together in a jar (a big jar) and the jar is closed. After long period of time what will be the mole fraction of solute in each beaker ?
I have given the last question from a book...the solution is given in the book but i dont understand it ..can someone solve and explain it to me?
-
UP 0 DOWN 0 0 16

16 Answers
Ans-1. When we add a non volatile solute to a solvent it decreases the surface area of solvent therefore boiling point increases and freezing point decreases.. OR due to -ve deviation, freezing point decreases..
When we add a non volatile solute it decreases the surface area of solvent. Particles of solute doesn't dissolve in solvent so the solution become thick therefore it will freeze at low temperature..
when u add non -volatile solute to a solution then particles of non-volatile solute get fixed in the solvent and covered some of the empty space in the solvent and as a result the solution becomes thick and resultingly there is a depression in freezing point. we can also say that the kinetic energy of the solvent particles decreases due to the space occupied by the solute molecules
u see ...the freezing of a solution depends on the vapor pressure above it
on adding a non volatile solute, we decrease the vapor pressure above the solution which lowers the freezing point ....
we know that a liquid becomes solid at different temperatures for different pressure
when we add a non volatile solute to a solvent, it doesn't dissolve in the solvent and decreases the surface area of solvent therefore the solution will become thick (thick solutions have low freezing point) therefore its freezing point decreases...
Due to -ve deviation, vapour pressure decreases therefore boiling point increases and we know that B.P. is inversely proportional to freezing point therefore freezing point decreases..
ok guys thanx i guess i have undestood this thing
please try the other two also
this one is a highly concept building question from osmosis...plz try to answer this cuz im not able to get the answer
Q-'Time Release' drugs have the advantage of releasing the drug to the body at a constant rate so that the drug concentration is not high enough to have harmful side effects or so low as to be ineffective.A schematic diagram of the pill that works on this basis is shown below.Explain how this works so as to keep drug release constant?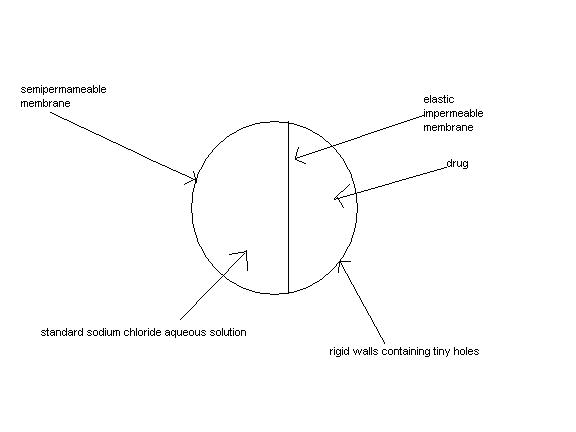
in the question 2 i m asking bout increasing external pressure like this:
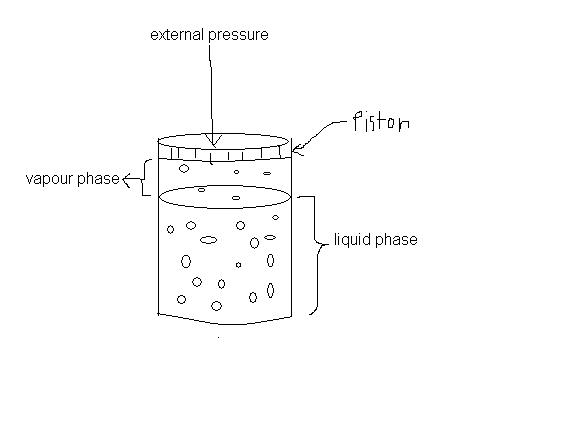
@all people here
is there no one interested in solution chapter here...
for the sake of physical chemistry plz help[36]