hey, can u plz increase thesize of the image???
the firgures r difficult to be read...
Consider a pH titration of 50 mL 0.1 M benzoic acid vs 0.1M NaOH at 25°C. The experimentally observed curve is shown below, on which points A, B, C…… F are marked.
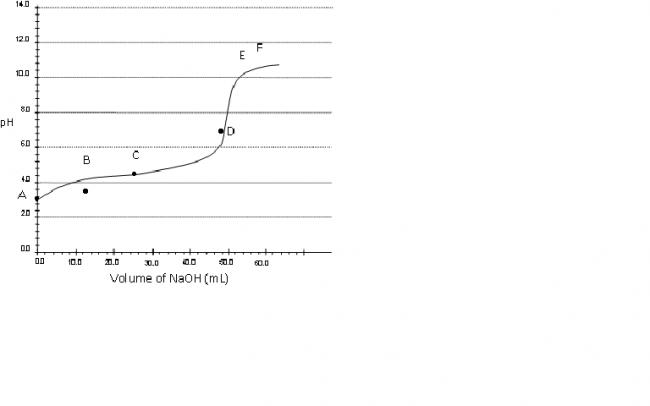
a) Which point corresponds to approximately 0.07 M
benzoic acid solution?
b) Suppose you are carrying out a conventional titration of benzoic acid and NaOH. Using choose an appropriate indicator from the table below.the answer obtained
Indicator pH range Colour change
Methyl yellow 2.9 - 4.0 red-yellow
Methyl orange 3.1 – 4.4 red-orange
Phenolphthalein 8.0 - 9.8 colorless-red
hey, can u plz increase thesize of the image???
the firgures r difficult to be read...
but just have a look at the point B and think just u'll understand.......
atkeast gimme sum hints to figure
nywz i dnt think so much typin is reqd here...
ok deepan.......u ask eureka 123........he is targetiit minister he will explain the details........