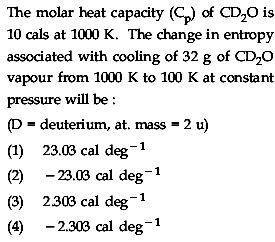
1 Answers
Sourish Ghosh
·2014-04-28 11:36:17
The given unit of Cp is wrong I think. It should be cal/mol-K.
ΔS = ∫dQT
→ ΔS = ∫nCpdTT
→ ΔS = 2.303x10xlog1001000 [ n = 1 ]
→ ΔS = -23.03 cal/K (or cal/deg)
 Aditya Agarwal Thanks! :)Upvote·0· Reply ·2014-04-28 12:34:30
Aditya Agarwal Thanks! :)Upvote·0· Reply ·2014-04-28 12:34:30