Plzzzzz reply......
The graph shows titration curve for diprotic acid using standard NaOH soln
Which of these is correct
a)vOL A) represents 1st end point
b)pH B) represents end pt at pH=7
c)pH D represents half neutralization pH that maybe used to estimate Ka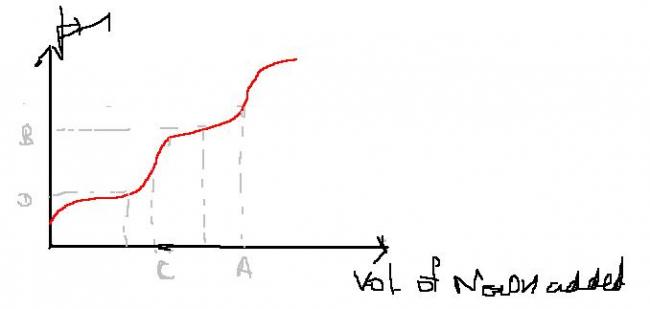
-
UP 0 DOWN 0 0 9

9 Answers
msp
·2009-01-24 20:12:50
the point C must give the EQUIVALENCE point of the first reaction that is loss of the one proton in the molecule and i think the corresponding pH shud be the half neutralisation of the diprotic acid. Please correct me i know dat i was wrong