wats ur doubt here?????
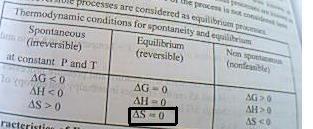
for a process in equilibrium ΔG=0
ΔG=ΔH - TΔS
THEREFORE ΔH=TΔS
ALSO FOR A REVERSIBLE PROCESS IN EQUILIBRIUM ΔS=0
WHICH IMPLIES THAT ΔH=0 FOR ALL PROCESSES IN EUQILIBRIUM????
SOMEBODY PLEASE EXPLAIN
-
UP 0 DOWN 0 0 13

13 Answers
yup...... of course all parameters(del s,delH,delG==0 AT EQUILLIBRIUM...)

dis s clausius.....
contact no:9894543210
email id:clausius@gmail.com
orkut profile link:http://www.orkut.co.in/clausius#Profile.aspx?
contact him fr any queries abt thermodynamics.....
"ΔS=0 at equilibrium"
^^^have u heard this anytime b4, sky?? this is the first time i'm hearing such a thing
me too..
arey clausius statement ofcrz everyone has heard ... but this man is so easily available kya?? [50]
and he is still alive [50]
i mean us no. pe phone karne se clausius will talk to us ?? [50]
ALSO FOR A REVERSIBLE PROCESS IN EQUILIBRIUM ΔS=0
what do you mean by that??
If a reversible isothermal process is in equilibrium implies that expansion and compression are taking place at same rate
i.e. state of the system remains unchanged
then obviously ΔU=ΔH=ΔS=ΔG=0 as they are state functions