let me show you the solution given by the book which i didnt understand,may be that will give some idea
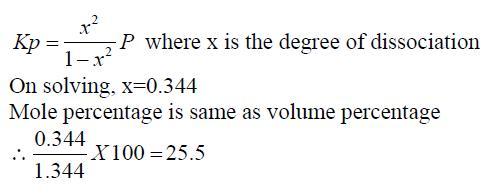
The volume percentage of Cl2 at equilibrium in the dissociation of PCl5 under a total pressure of 1.5 atm is? (Kp=0.202)
answer:25.5 %
what does volume percentage means!
i thought that any gas occupies the whole volume and it is always 100% :O
let me show you the solution given by the book which i didnt understand,may be that will give some idea
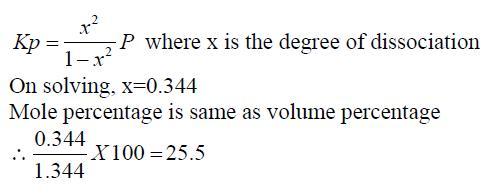
if you assume that the reaction is taking place in gasseous state, then
take initial partial pressure as P
dissociation alpha
P(1-α) Pα Pα
then the thing you have done follows!
lol new complication !
take is as a gas for time being..just show me how you solve those kind of problems !