@madhu
K3CrO8 is a complex K3[Cr(O2)4] in which Cr is in +5 state
In alkaline soln K2Cr2O7 with 30% H2O2 gives a red brown compound A(K3CrO8).Find oxidn state of Cr in it.
-
UP 0 DOWN 0 2 8

8 Answers
check the following links...
http://en.wikipedia.org/wiki/Potassium_tetraperoxochromate(V)
http://cat.inist.fr/?aModele=afficheN&cpsidt=951392
These kinda questions are only meant for students to lose marks..
thx govind....
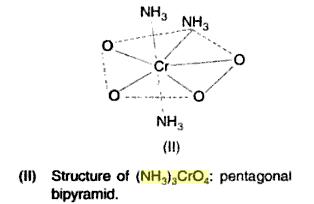
and ya that NH3 ques was also there in passage..though that was easier[1]
got a very nice way of finding the oxidation states of such compounds
Assume the number of peroxy linkages to be y and let the oxidation state of the metal be x ...
as the compound is neutral
so we can write
3 + x + y(-1) + (8-y)(-2) = 0
on simplifying it we will get
x + y =13
now the number of peroxy linkages shud be even...
so possible values for x and y are
x y
11 2
9 4
7 6
5 8
since the oxidation state of chromium cannot exceed 6 so first three cases are not applicable..and moreover the number of peroxy llinkages cannot excced 8 so y cannot be greater than 8..
hence the oxidation state of chromium shud be 5
plz comment on the abv method...
and experts plz do tell if this method has any exceptions...