bcos H+ is a smaller cation.also HCL is a strong acid compared to NaCl so i think its slope shud be gr8er than that of Nacl
Na+ is a larger cation compared to H+
A graph plotted b/w molar conductivity of NaCl,HCl,NH4OH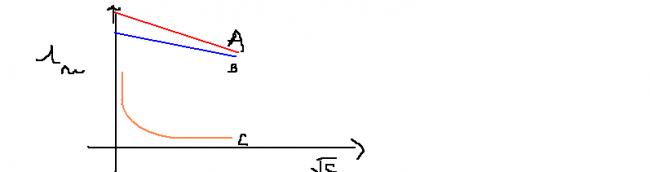
identifyA,B,C
i have done the identification job
A=HCl,B=NaCl,C=NH4OH
but wanted to know why slope A and B are diff ????since both NaCL and HCl are 1:1 electrolyte...??
-
UP 0 DOWN 0 0 7

7 Answers
but i have some dbt.
in conductivity see in case of H+ the cation is so small so it is heavily hydrated na.So the resultant hydrated molecule is large so its conductivity in soln shud be less than NaCl.
I was saying that concentrated HCl is not fully dissociated at high concentration. while NaCl is fully dissociated at high concentration
this may help you
why HCl not disociated completely..considering that it is strong acid...
I will try to find an appropriate answer for this
right now I don't have any