@ RAM,
BHAI,GRAPH B/W RATE(R) AND TEMP.(T).FROM WHERE THIS k CAME IN?????????
plot a curve depicting the temperature dependence of the rate R of a simple one step reaction.
-
UP 0 DOWN 0 1 24

24 Answers
Ramkumar and john..
I made a mistake...
Ramkumar's graph was correct...
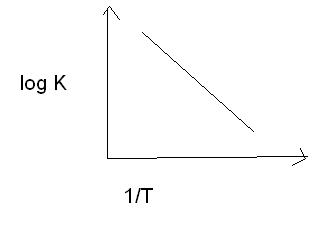
See the thing is that the exponential power is -Ea/RT
as T-> 0 kelvin, -Ea/RT goes to - infinity
exp (-Ea/RT) goes to zero...
yup,i know that but in answer the same graph starts from origin,plz explain
plot a curve depicting the temperature dependence of the rate R of a simple one step reaction.
this is ur q 1
u mean 1 step
so 2nd order kyaaaaaa ?
DUDE ! UR Q IS INCOMPLETE
TER R MANY RATE REACTIONS !
EXO OR ENDO ??
then 2 graphs possible !!
srry john.. pretended it as rate constant.......... very srry.......
wait let me post d correct one....
john..........
whom are u sying that?to me or ram???????
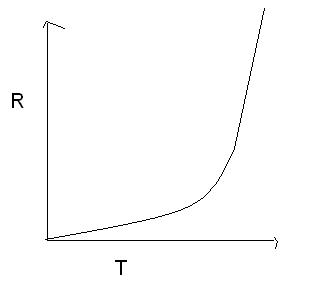
my graph is rate v/s temperature
hey.. then wat does ur q mean???????
temp dependance means?????????? think of it..........
q is how does k vary with respect to time...........
srry fr editin after pinkin.. got a small mistake.. corected now
A----->B+C
rate=k[A]m -----(1)
accc to arrhenius eqn
k=Ze-E/RT
put in eqn 1
=>Rate=Ze-E/RT[A]m
=>Rate=peT where p is constaant
made a change here
HEY,I NEED GRAPH BETWEEN R(RATE) AND T(TEMP.) FOR A SIMPLE ONE STEP REACTION. CHECK UR GRAPH.....WHY TAKEN K(y) AND 1/T(X)
HINT:acc. to answer(i am not able to solve) given graPH IS NOT A STRAIGHT LINE.IT IS A CURVE.
MAY BE THIS HELPS.
i mean it varies with 1/T with havin values in y axis as 0