4
4Hydration of the carbonyl group is least effective in the case of
(A)chloral
(B)glyoxal
(C)acetaldehyde
(D) 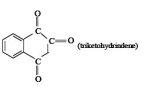
ANS D
 4
4A certain volume of ethylene after partial reduction with hydrogen in presence of a metal catalyst required 10% more oxygen for complete combustion than it would have originally. Calculate the ethane: ethylene ratio after partial reduction.
3 : 2
2 : 3
7 : 6
6 : 7
 1
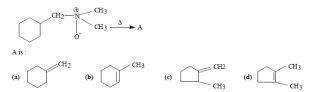
1q3 hofmann elimination answer A
 4
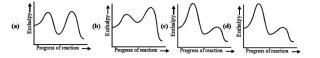
4For (1)
If the curve is steeper it means irreversible no ?? ( I am somehow confused over this)
Cud u plzz explain 1
For (3)
r8 ans
Thanks
 4
4ok fine
Any tries for 2 n 4 th qs ????
 39
39I think hydration is least effective in case of chloral...it would cause SN instead of nucleophilic addition.
 4
4No Pritish ans given is D for the second one
 3
3FOR 4 ITS COMING TO BE 3:2 ?????????
 3
3C2H4 INITIALLY LET a moles
C2H4→→C2H6
a(1-x) ax
C2H4+ 3O2→→2co2 +2H2O
C2H6 + 3.5O2→→2co2 +3H2O
total moles of oxygen used after partial reduction=3a+0.5ax(i.e 3a(1-x)+3.5ax)
now 0.5ax=(10/100)*3a
so x=3/5
reqd. ratio = x/1-x
that is 3:2
 1
1Q1........
if steeper the curve, higher is the activation energy, slower is the rate of reaction.
Therefore, ans has to be either of c or d, but c n d r lukin identical.....aren't they?
if v luk at de graph c n d.......Ea of reactant and Ea of intermediate is nearly same, indicates that the rate of forward reaction is equal to rate of backward reacion....... means reation is at equilibrium.......
 1
1Q2.......
ketones are less reative than aldehydes.........
 1
1Q4......
I think rocky is rite.......