yeah thta is wat i did bt a bit mistake
c d formula is K1=(xe/at) ln(xe/xe-x)
wer xe is equalibrim conc
a initial conc
This reversible reaction is 1st order.
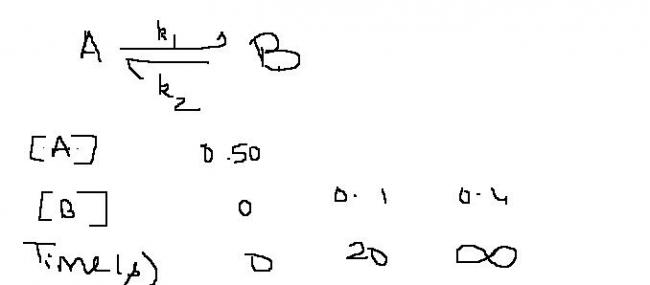
Q1 Calculate rate constant for forward direction.
Q2The concentration of reactant remaining after 1hr is?
Q3Draw curve of concentration v/s time of A and B simultaneously.
yeah thta is wat i did bt a bit mistake
c d formula is K1=(xe/at) ln(xe/xe-x)
wer xe is equalibrim conc
a initial conc
integration... can u plz tell me the page no and the book from where u got this formulae or if possible can u make a picture of thet and post it i have been trying to solve the equation formed but the diffrential eq i formed i was not been able to solve that plzzz posts
ya itz ohk bt iit is objctive y d hell sumone sit n derive d formula der
integration... dont feel bad.. but i dont like it sometimes when you do it formula based.. *(sorry!)
Can someone prove this "Formula" that integration gave?
i cant understand the question. where r the other concentrations of A?
sorry eureka getting no clue at all not been able to solve the differential eq form for the general value of n (n is the order of forward eq ) plz can u post ur soln