ya its fine...thanxxxxxxxx
atomic sold is made up of A type of atoms occuying BCC posiitons.A guest atom of largest possible size without disturbing original config is palced at (a/2,0,a/4) .where a=side length of cube.
Find packing fraction
-
UP 0 DOWN 0 1 2

2 Answers
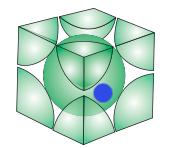
the blue colour circle shows the position of the guest atom...
i took the centre of the lattice at (0,0,0)..
so using the relation that √3a = 4r
where a is the side of the cube and r is the radius of the atom
and then some trigonometry i got the value of radius of the largest possible guest atom that can be accomodated is 0.13a or 0.30r
then i tried to calculate the packing fraction got the value using calculator.... it comes out to be 68.45%..since half of the guest molecule will be inside one unit cell... : )