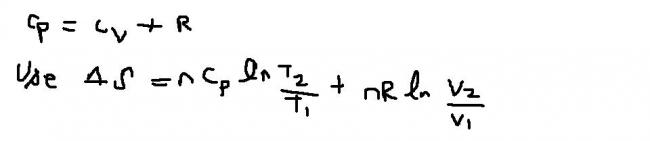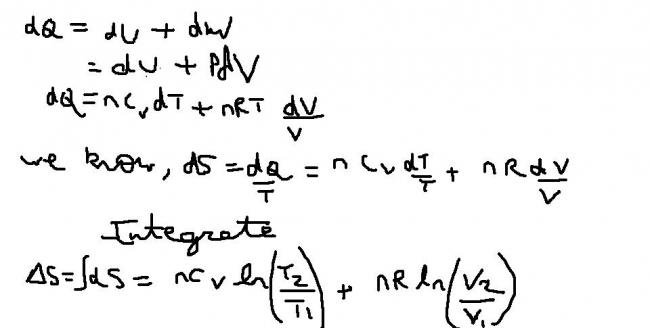this is general formula.............conditions dalo aur expression change ho jayega.............[1][1]
something is definitelly too wrong or too right today
2 questions in chem that too same topic [4]
plz help guyz and galz
Q) Three moles of an ideal gas (Cv = 12.5 JK-1mol-1) are at 300K and 5dm3. If the gas is heated to 320 K and the volume changed to 10dm3, calculate the entropy change .
[7][7]
-
UP 0 DOWN 0 1 21

21 Answers
[7][7][7] [2][2][2] plz help people...
the process is not adiabatic or isothermal or isobaric so wat to do...
should i derive it for u??????
use first law for reversible expansioin.......
i can do it if u say.......it would take a minute for me to think about that.......[1]
any background abt the formula eureka or any comments you'd like to make
sory i didnt see this post before.....otherewise reply would have come lot before................
its easy if u know the formula..