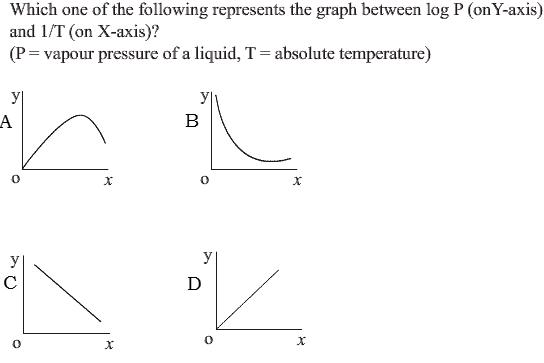
some1 pls ans it
4 Answers
Asish Mahapatra
·2009-05-05 20:00:02
as P is the equilibrium constant of the equation H2O(l) -><- H2O(g) Now, ΔG0 = -RTln(K) where K=equilibrium constant ==> -RTln(K) = ΔH0 - TΔS° ==> lnK = ΔS°/R - ΔH°/RT So, taking logP = log(K) as y and 1/T as x we have, y = c - ΔH°Rx So, it is a straight line. If ΔH°>0 then slope is negative and if ΔH°<0 then slope is positive. This reaction is endothermic. So, ΔH>0 hence slope -ve. Hence answer is (C) cheers !!