Yeah agree with rickde...molecularity and order of a reaction are unrelated.
S1 : For the reaction :
O3 \Leftrightarrow O2 + O
O + O3 \rightarrow 2O2 (slow)
---------
2O3 \Leftrightarrow 3O2
The molecularity of first step is 1 and that of second step is 2.
S2 : O(g) is an intermediate and rate of reaction is K[O3]2[O2]-1 and order of the reaction is 1.
-
UP 0 DOWN 0 0 6

6 Answers
@Asish : Yes molecularity can also be defined for a reversible reaction
This is a reversible bimolecular reaction
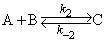
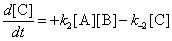
@Ronald : molecularity is the term which tells us the number of molecules involved in an elementary reaction step.
sequence of elementary reaction steps together to form what is called a mechanism
So the qs is a mechanism given in which molecularity of each elementary reaction step is asked for
I too go with B