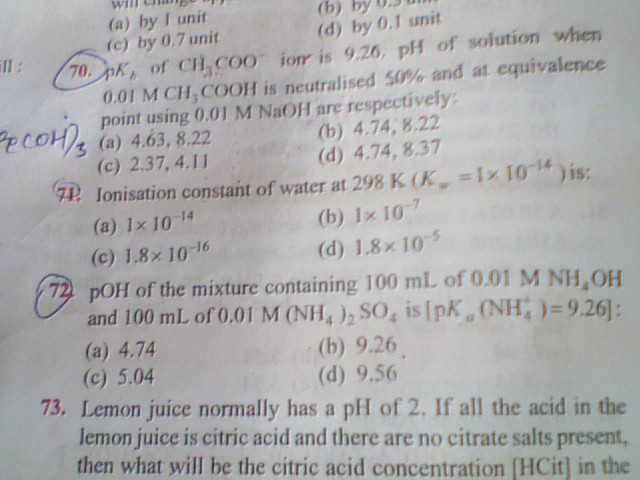arey haan shit ..........sahi yaar yaar
IONISATION CONSTANT poocha hai
11 Answers
Mani Pal Singh
·2009-03-28 07:57:30
yaar humein copy mein kuch Ka yad rakne ke liye kaha tha
uun mein se yeh ek hai :P
will try u the method after some time
Asish Mahapatra
·2009-03-28 07:59:27
Q71 .. H2O -><- H+ + OH-
[H2O] = 55.5 moles/litre.
Now ionisation constant = K of equation = [H+][OH-]/[H2O] = Kw/55.5 = 10-14/55.5 = 1.8*10-16
°ღ•๓ÑÏ…Î
·2009-03-28 08:08:38
Ragadeepika
·2009-03-29 08:11:58
72.
The solution clearly forms a buffer.
Using henderson equation -->
pOH = pKb + log \frac{[salt]}{[base]}
[salt] = (0.01 * 100)/ 200
[acid] = (0.01 * 100)/200
pKa + pKb = 14 {for a particular conjugate acid-base pair}
pKb = 14 - 9.26 = 4.74
pOH = 4.74 + log 1 = 4.74
Thus, answer is A.
Please correct me, if I'm wrong.
ALL THE BEST
°ღ•๓ÑÏ…Î
·2009-03-31 09:53:59
i dunno i 2 gt d same answer bt woh answer ni hai
can u try d first q also