m gettin it as 47. 1 ...............may b calc mistake wait will try agin
An optically active isomer of A is dextrorotatry.A on dissolving in organic solvent starts isomerising into leavorotatory form by first order process as
A(+)→A(-)
The data for progress of reaction is :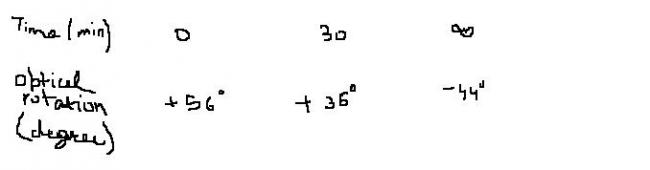
Answer these on teh basis of above info.:-
Q1 What is half life of isomeriszation process?
Q2 What will be the optical rotation at half life
Q3 WHat will be optical rotation of soln after 1 hr
-
UP 0 DOWN 0 0 12

12 Answers
k=[2.303loga/(a-x)]/t
a=56
a-x=36
t=30
u egt k
itz fisrt order kinetics
so t(1/2)=.693/k
u can find rest
me too something in this range...........but answer is 90 minutes.........
a=rinfi -r0
a-x =rinfi -r t
they cum out 2b negative bt as both cum 2 b negative it gets canceld
r infi means rotation at infinity
r 0 means rotation at t=0 and rt means roration at t u can refer arihant 4 dat itz a solved eg (similar type)
r infi means rotation at infinity
r 0 means rotation at t=0 and rt means roration at t u can refer arihant 4 dat itz a solved eg (similar type)