13
13Am in full sleeping mode right now...so dont mind if there's a terrible blunder :P
(two Methyl(s) fr the 1st structure)
 13
13And yes, better logic fr b)...better than R-S.
Thnx fr reminding. ;)
 13
13Arrey i posted in Instant Chat...u didn't reply...phir yahaan kiya :D
Ok, Mech.- (Following Diazotisation first),
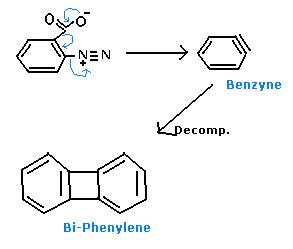
I feel like hitting myself now, phir nahi dekha ki u edited :'O ... anyways, ab bana diya tha, toh am also posting :P.
(Thoda jaldi bata diya kar ki u're about to post the same :P)
 39
39What took you so long? lol..
There is no decomposition. 2 mols of benzyne combine to form biphenylene.
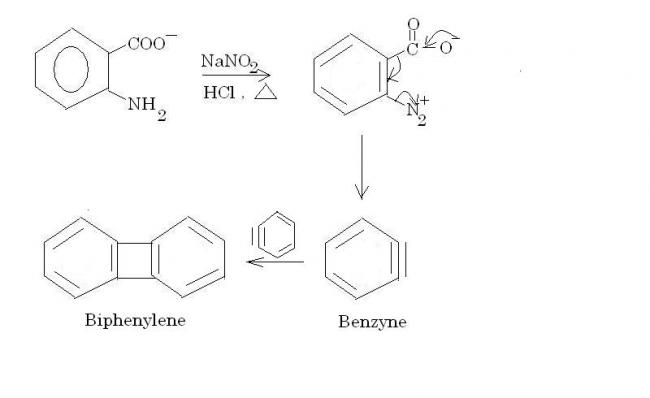
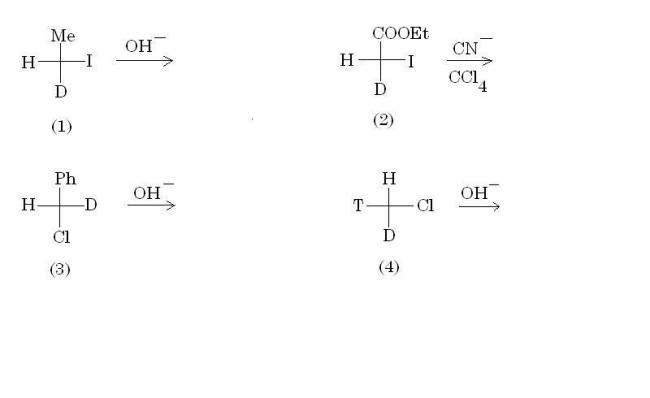
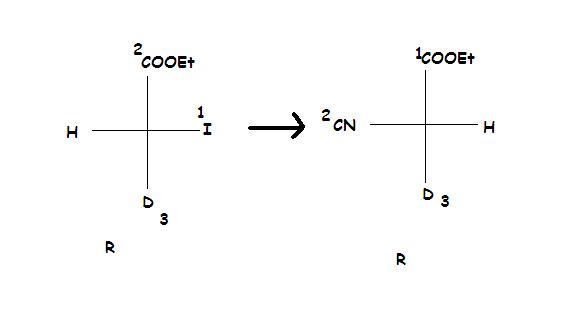
And for part (b), remember this rule regarding SN2.
When the priority order of the incoming nucleophile and the leaving group is the same, then only inversion of absolute configuration takes place. This doesn't happen in option (2), and hence the configs are the same as subho pointed out.
 13
13Can only think of a Bi-Phenylene beyond Benzyne....anything else ?
 39
39You're close Avik...quite close :P
 39
39Lol...I posted the question wrongly. Tum sab mera sar kaatke rakh dete agar main theek se sabkuch batata nahi :P...waise bhi kya pata TS Nattu kahan chchupa hua hoga warna kitne saare bihari uska sar kaat dete :P
I posted another question which is related to aryl halides as 14th ain't over yet like Avik said...try it.
 13
13Arrey sry, didn't see u edited to post the Mech. :'O
But u cud have waited na, i was working :( ....14th aint over yet.
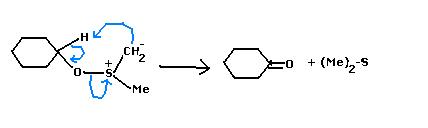
(Ok, Mine is an Alternate Mech. :P (based on which "H" the base takes....i took away the methyl "H" 'coz DMSO methyl are slightly acidic.)
 13
13Yes, the base was necessary.
After the sulphoxonium intermediate & action of base, this is wht i do now...
 39
39Nahi...as subho made me forget the mechanism, I was also trying that now hold your guns and knives...torture him later :P but my apologies avik, the mechanism follows an oxysulfonium intermediate...THEN the ketone is formed!
You were right about the oxysulfonium salt. Now proceed to the answer :P
(Isliye never delete your answers :P)
I shall explain :) you are right only partly in your latest post! It is confusing to both you and me...
The paper from where I got the question...the question is indeed incomplete. The missing link is the presence of a basic medium.
How farcical was that :P worse than JEE lol.
In any case..I think I owe everyone an explanation to the answer. I am accountable to you all(unlike the JEE committee) so here is the answer -:
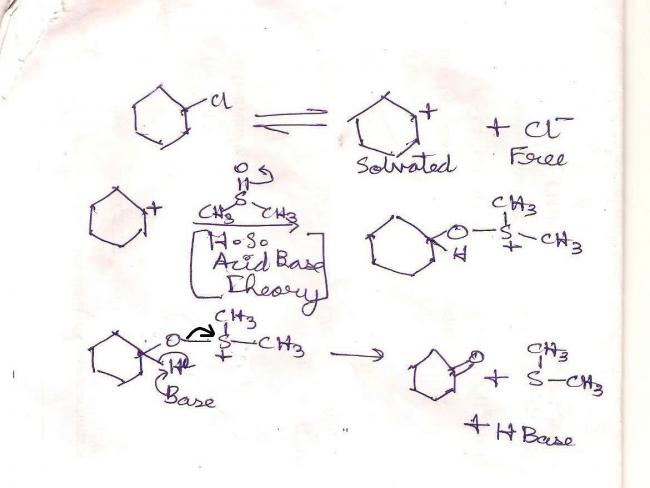
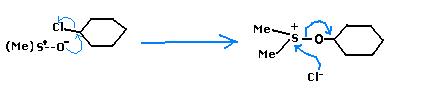
Step I : DMSO's solvent property comes into play. The cation is well solvated but the chloride anion is free. This is because of the low charge density on the sulfur atom(+I effect of methyls).
Step II : Oxygen being a hard base and a carbocation being a hard acid, join hands. This is the oxysulfonium "salt" intermediate(chloride anion is free, note, so it plays no part in the reaction).
Step III : The positively charged sulfur atom leaves, which in turn causes a hera pheri of bonds. Simultaneously the basic medium abstracts a proton so that oxygen can form a pi-bond with cyclohexyl carbon. (The sulfur part leaves, oxygen becomes positive, base abstracts proton, pi bond is formed between (-)C and (+)O)
End products are as you can see. This reaction is a reaction characteristic of DMSO. It can oxidize such organic chlorides(and is itself reduced to DMS). If the chlorides are converted to tosylates the job becomes easier for it.
Avik, as you have atleast posted one half correctly(even though it is not what actually happens), and mentioned the oxysulfonium salt, I have given you a pink as compensation lol.
 49
49quest 2...it is the 2nd one!!!
 39
39Abey yaar lo...ketone banega. Now find out how. :P
 13
13Not rite ?
Give a small hint then.
 13
13CycloHexanol mein se H+ hata kar :P (Am trying some random mechanisms :P)
 39
39Structure please...mujhe yeh sab samajh nahi aata :P
 39
39Yes it is correct rocky :)
No that's incorrect avik :P
 3
3similarly for second one...........
3.2 x 10-5 [2-bromobutane][HO-] 3.2 x 10-5 [2-bromobutane][HO-] + 1.5 x 10-6[2-bromobutane]
3.2/1.532=2.088%
 3
3for SN2 rekn.
fot first case % of this reaction takes place by the SN2 mechanism =
3.2 x 10-5 [2-bromobutane][HO-]3.2 x 10-5 [2-bromobutane][HO-] + 1.5 x 10-6[2-bromobutane] *100
=320/335*100=95.522
 3
31..95.5%
and 2.088%
is it correct????????
 39
39Gallardo it's not the fourth...and rocky SN2 sabmein ho raha hai :P
Of course you can use the long and boring way, to find R-S config in both reactant and product(as subho is about to do), but there is an easier way I will discuss later :P it may save 5 seconds of your time!
 3
3well for 2 it seems second one ..SN2 rekn.