 1
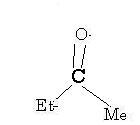
1its a very close call in deciding which has more migratory aptituide
ethyl or methyl
but u can see there are two methyl groups in comparision two one ethyl group
when there is closesness in migratory aptituide
we go for major product
so the methyl group migrating will be the major product (as it has greater chance)
correct me if i am wrong !
 1
1for #18
first edited.. then you posted. :P
 39
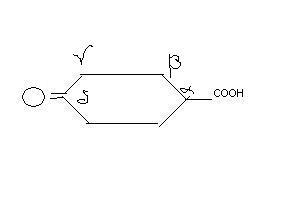
39@Post #15, you have misinterpreted my statement about beta-keto acids. Nobody asked you to look at the ketone group which is formed by hydrolysis of the acetal.
There is no such thing in Morrison and Boyd. Blanc's rule for the heating of 1,3-dicarboxylic acids states that the loss of CO2 occurs, and occurs by a cyclic transition state(sounds familiar?).
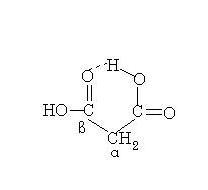
But good that you understood...and later edited :P
 39
39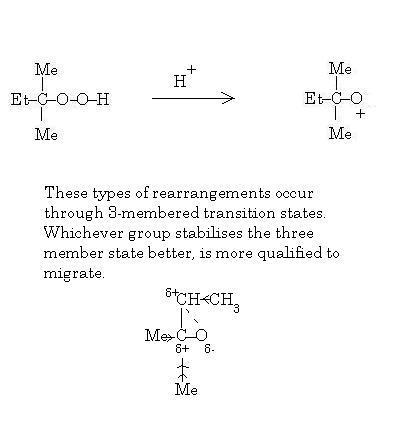
Not rearrangement actually..migration I mean. And that's CH2CH3
 1
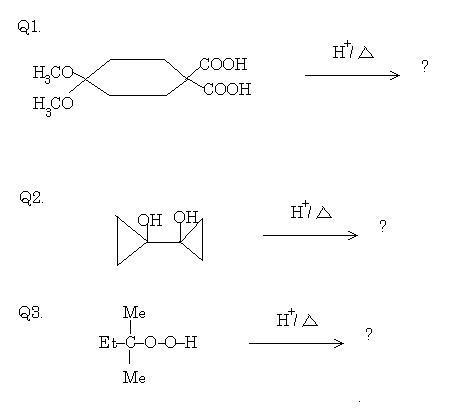
1For Q2:


err... entire courtesy to Pritish Chakraborty (including images).
PS: @Pritish: I was not being adamant. I was just trying to check out my basics and get them correct in case they were wrong. :P
 39
39@going ahead : I could explain everything right now, but Ankur's being adamant and wants to explain himself first..he's having lunch. Let His Majesty finish his royal luncheon and then I'll see..haha.
 1
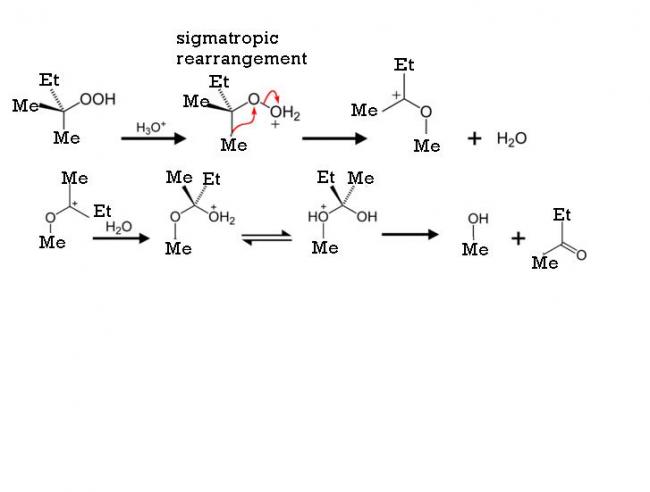
1ankur post the mechanism please
 39
39The mechanism is perfect....but you have not taken into account migratory aptitude!
 39
39Yes it is indeed by the cumene hydroperoxide method. You're nearly there.
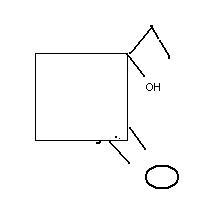
Just to clarify, in Q1, those are ether groups attached on the left side of cyclohexane, not carbonyls.
 1
1and an alcohol
my working is simillar to production of phenol by cumene peroxide method
yes i think some ammendment is required
please wait
 39
39Show your working in Q3 bhai...you are nearly there if you're following the right line of thinking. There will be two products however.
 39
39Incorrect answer, but nice try souvik!
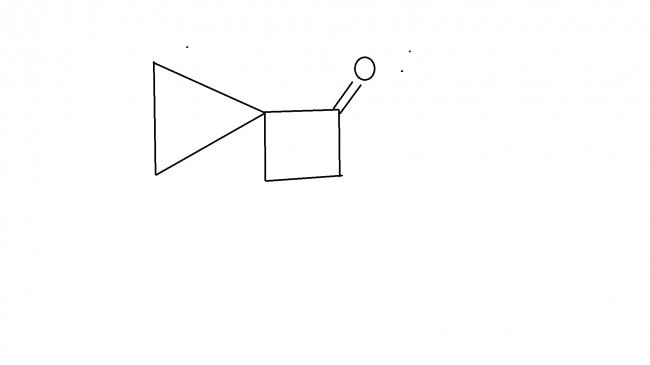
A little hint for you. The cyclopropane group is extremely acid labile. It may look like pinacol rearrangement, but it's not.




 ??
??