yeah i guess..practice is evrything
Classify the 10 compounds given below into three groups:
a) Aromatic.
b) Anti-Aromatic.
c) Non-Aromatic.
Mention reason for every grouping! [1]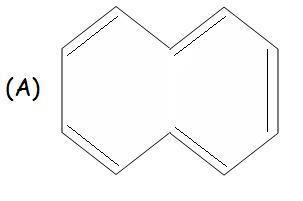
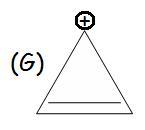
-
UP 0 DOWN 0 0 19

19 Answers
That is a matter of practice and exposure Debosmit...
Nothing else makes u perfect in organic chemistry.. [1]
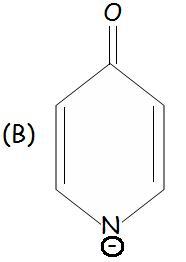
B is aromatic because one of it's resonating structures have same 3 double bonds like benzene...wid a O- as a substituent!
ok.thnx a lot subhomoy da.
but i hv a qstn sir....
how do we know which molecule will have steric hindrance n which which wil not?so we have to analyse the structures of the molecules pretty thoroughly then....
For A..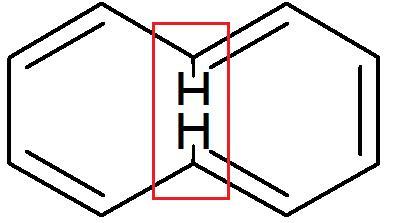
The two H as shown, face vaderwaals repulsion from one another (Strric hindrance)
and thus to reduce the repulsion(in order to reduce energy) one of the H moves up a bit and the other moves down..
This causes the molecule to lose its planarity and thus, it becomes NON AROMATIC.
If we try to hydrogenate benzene, using normal conditions of hydrogenation, we would see that the hydrogenation wud not take place though we would expect 3 moles of hydrogen to be used up for each mole of benzene (3 double bonds)
The reason for this is the three double bonds in benzene participate in delocalisation.. and thus immune to hydrogenation..
Thus, any pi-electron pair that participates in delocalisation is immune to hydrogenation.
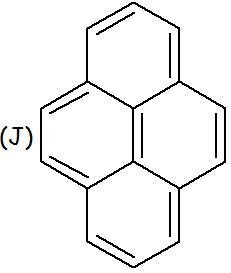
In case of (J)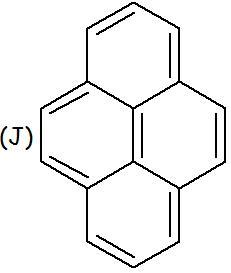
If we try to hydrogenate using normal hydrogenation conditions, we would see that ONE MOLE OF HYDROGEN is being consumed per mole of the compound..
Which pair gets hydrogenated? Which lone pair is not getting delocalised??
It is this: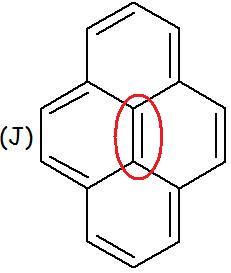
why?
because, aromaticity provides most stability in organic chemistry...and everything in this world drools for stability and this compound is from this world only..
Thus it becomes stable by not delocalising one electron pair..and all the peripheral pairs start a delocalisation current! [1]
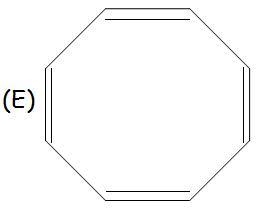
E has a structure like this: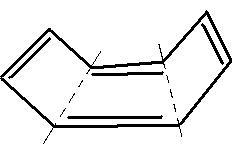
Non-planarity is the reason for non-aromaticity!
in E, due to repulsions and hindrance due to pi electrons, it bends and forms a tub structure, as it goes out of plane it can not undergo resonance and hence is non aromatic.
in J, only the peripheral pi bonds take part in delocalisation and hence the central pi bond is not included in the no. of delocalised electrons and is therefore aromatic.
in case of E,all the carbons are sp2 hybridised but why is it not planar?
and in J cnt undrstnd the delocalisatn part....rest are ok..
(A) (B) (C) (D) (F) (G) (J) Aromatic
(E) (I) Non - aromatic
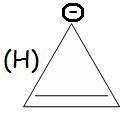
(H) anti - aromatic
My first organic post in over a year [1]
i remember nothing now [3]
pta hai .. but still i categorize as a baccha now .. having forgotten evrything ... waise bhi is sem mein kaam aayega [3]
A is again non-aromatic because of steric effects as it cant easily obtain a planar shape
C should be aromatic because of 6 delocalised electrons
same with B i guess [7]
we have chem in sem 3 [2] ...
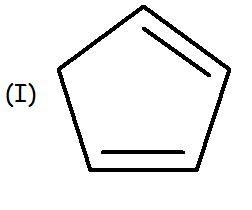
I is non aromatic ... no delocalisation
same for E as it is not planar in shape rather tub shaped.. so delocalisation not possible. hence non-aromatic
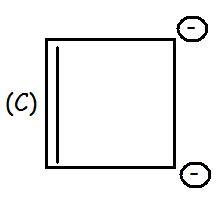
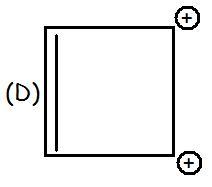
H is anti-aromatic bcz of 4 delocalised electrons (4n)
G/D is aromatic bcz of 2 delocalized electrons (4n+2)
J aromatic bcz the middle double bond does not participate in delocalisation .. so there are 14 delocalised electrons (4n+2)
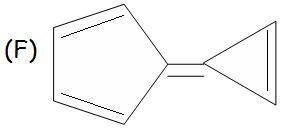
F should be aromatic (if you shift the double bond joining the two parts such that u get cyclopentadiene anion and the other cation .. then both parts are aromatic)
sure of these [1]
rest all were my hunch
Aromatic Anti-Aromatic Non-Aromatic
A,C,D,F,G B,E,H I,J
i`m nt sure about J....it may be anti-aromatic