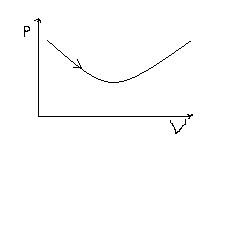
p-V diagram of an ideal gas enclosed in a container is shown in fig. How the work done by the system on the surrounding is changing i.e., whether it is continuosly increasing , decreasing etc?
-
UP 0 DOWN 0 0 5

5 Answers
Subhomoy Bakshi
·2010-03-13 07:12:56
work done is +ve and it is continuuously increasing obviously!![1][1]
Bicchuram Aveek
·2010-03-13 07:44:59
work is +ve because volume is increasing in the process. (and also because work is done BY the SYSTEM )
Subhomoy Bakshi
·2010-03-13 07:52:29
look on any P V curve....thus, if the line is moving in clock wise direction then work is done by the system and it is positive!![1][1]