Thanks Nishant Sir & Rahul
MULTIPLE ANSWER QUESTION
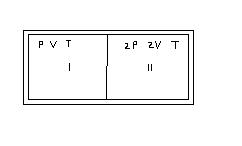
1) A partition divides a container having insulated walls into two compartments 1 and 2 .
The same gas fills the two compartments whose initial parameters are given.
The partition is a conducting wall which can move freely without friction. Which of the
following statements is/are correct, with reference to the final equilibrium position ?
A) The pressure in the two compartments are equal.
B) Volume of compartment 1 is 3v/5.
C) Volume of compartment 2 is 5v/6.
D) Final pressure in compartment 1 is 5P/3.
-
UP 0 DOWN 0 2 4

4 Answers
A,B,D
Final pressures should be equal.
and apply PV=constant for both compartments
Pressure will be same because you want the wall to be at rest and not accelerating..
So if the volume is x on the Left side while it is 3V-x on the right side..
Also, on the left side,
P1.x=PV
P1.(3V-x)=2P.2V
Dividing, we get 4x=3V-x
so x= 3/5V
Pressure is 5/3P